
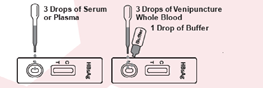
The test is suitable for laboratory testing and screening using venipuncture whole blood, serum, or plasma specimens. CE marked as an in vitro diagnostic device.
Easy-to-Use
• Simple procedure format1. Drop specimen sample
2. Add buffer
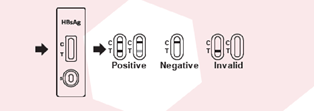
3. Wait for result• Rapid results within 15 minutes
• Rapid detection of HBsAg in venipuncture whole blood, serum, or plasma.
• Ambient storage and transport conditions: 2°C - 30°C (36°F - 86°F).
Accurate & Reliable
• Over 99.86% accurate:◦ Sensitivity: 99.87%
◦ Specificity: 99.86%• Includes a control line to confirm the validity of the test.
• Shelf life of 24 months from the date of manufacture
• Utilizing monoclonal and polyclonal antibodies to detect elevated levels of HBsAg in whole blood, serum, or plasma.
Accessible
• Competitively priced for large-scale programs.
• Short lead times
Various clinical stages, including latent, asymptomatic infection, are common in Hepatitis B (HBV). HBV is a preventable and curable viral infection primarily transmitted through blood contact. If left untreated, it can result in serious health complications, notably liver disease.
Training materials for our products are available and recommended to ensure proper utilization by healthcare professionals. These materials cover specimen handling, storage, and equipment maintenance, ensuring accurate results. Request more product information »
Product not available in all territories. Please contact us for details. Please read full instructions before use.
*OraSure provides information regarding reimbursement (including, in some instances, potentially applicable CPT, HCPCS and/or analogous state or local codes or designations) for background purposes only. It does not constitute legal advice or a recommendation regarding clinical practice. Information provided is gathered from third-party sources and is subject to change without notice. The provider has the responsibility to determine medical necessity and to submit appropriate codes and charges for care provided. Any decision regarding specific coding is at the discretion of the healthcare professional, and provision of this information does not guarantee or facilitate reimbursement. OraSure makes no guarantee that the use of this information will prevent differences of opinion or disputes with Medicare or other payers as to the correct form of billing or the amount that will be paid to providers of service. Please contact your Medicare contractor, insurance provider, other payers, reimbursement specialists and/or legal counsel for interpretation of coding, coverage and payment policies or specific billing questions or concerns. Nothing herein is intended to promote or facilitate the purchase or use of products outside of their approved or cleared indications, and appropriate use of products should be based on the healthcare professional’s medical judgment.