
Written by Jennifer Harris, MSc. 5/6/2024
Syphilis is a multistage sexually transmitted disease caused by a squiggly-looking bacteria, Treponema pallidum (T. pallidum). Although ~20 years ago syphilis was thought to be a disease of the past, a syphilis epidemic is occurring. In the United States alone, 203,500 cases were reported in 20221 , nearly double the reported cases of 2018.
Underserved Communities
Most of the increase in syphilis cases -- for all stages -- has been among men who have sex with men. However, there has been an alarming increase of cases in women and, correspondingly, in babies born with congenital syphilis due to vertical transmission from mother to child2 . Black/African American and American Indian/Alaska Native people and other traditionally underserved populations, who often face adverse social conditions related to healthcare access, are disproportionately affected. This overall trend has increased demand for testing that can expedite syphilis diagnoses and quickly link patients to care.
Nontreponemal and Treponemal Tests
Point-of-care (POC) tests for sexually transmitted infections enable more immediate results and treatment options. To fully explore the utility of a rapid test for syphilis it is helpful to understand the two serological test types -- nontreponemal and treponemal – both of which are required for a laboratory diagnosis and follow one of two test algorithms. It is the order in which these antibody tests are run that defines the algorithm.
Traditional and Reverse Algorithms
The Diagnostics Direct Syphilis Health CheckTM test has been added to OraSure's infectious disease testing portfolio. Manufactured by Diagnostics Direct LLC and distributed by OraSure, it is a lateral flow immunochromatographic rapid test that detects treponemal antibodies at the point-of-care. Labs have increasingly adopted the reverse sequence algorithm – conducting a treponemal test first -- over the traditional algorithm -- running a nontreponemal test first -- to meet exponential testing demands. Algorithms matter as they drive efficiency as well as diagnostic accuracy and consistency in clinical decision-making. (See Figure 1 for an illustrative example of the traditional vs. reverse algorithm workflows.)3
Compendium of Syphilis Testing Publications:
As the number of syphilis cases have skyrocketed since the 1950s, the demand for more immediate diagnoses and treatment to stop the spread of disease has increased. Amid a growing volume of evidence informing syphilis testing, we’ve selected studies most applicable to a point-of-care testing approach.
FIGURE 1. Algorithms that can be applied to screening for syphilis with serologic tests — CDC laboratory recommendations for syphilis testing in the United States, 2024
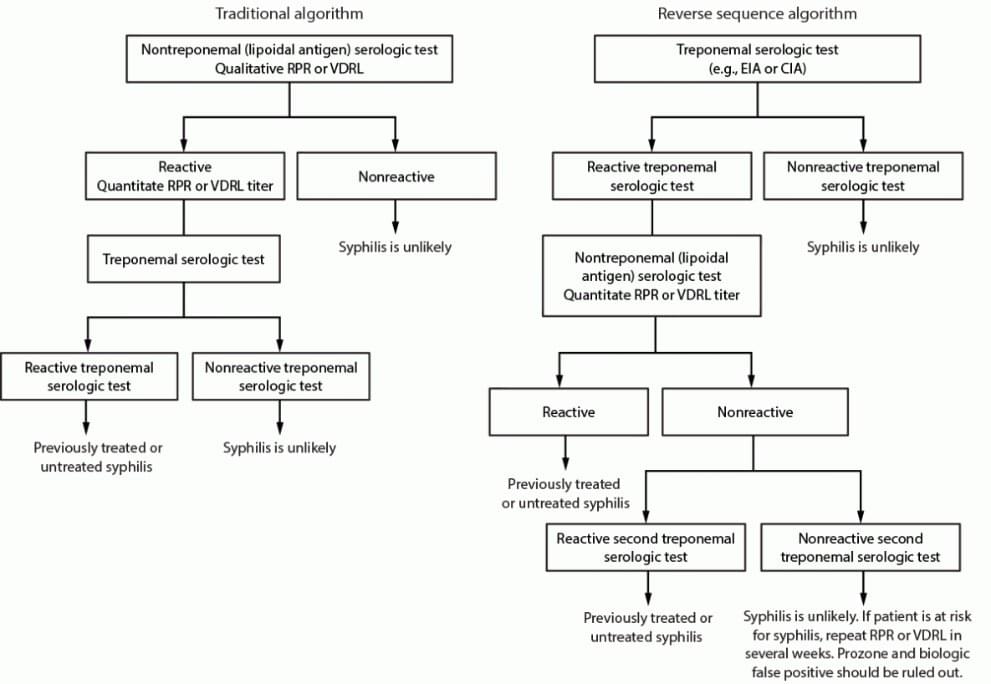
Abbreviations: CIA = chemiluminescence immunoassay; EIA = enzyme immunoassay; RPR = rapid plasma regain; TPPA = Treponoma pallidum particle agglutination; VDRL = Venereal Disease Research Laboratory.
Rapid Treponemal Testing
The Diagnostics Direct Syphilis Health Check Test can be performed on fingerstick whole blood to deliver results in 10 minutes in a CLIA-waived setting. This is an important innovation as rapid, POC treponemal testing is easily adaptable to meet local epidemiological trends and resource constraints. Healthcare providers can tailor testing and treatment strategies to meet the unique needs of a particular patient population.
American Indian Case Study
As an example, consider one hospital’s innovative response in rural Arizona. The Whiteriver Service Unit (WRSU) provides public health services and medical care to an American Indian community in Northeastern Arizona. The service unit observed a significant increase in syphilis from 2017 to 2021 that substantially outpaced national and statewide reports. The hospital served an American Indian/Alaska Native (AI/AN) population of more than 18,000 individuals. Within the hospital, workers tested 5888 AI/AN persons for syphilis whereby 555 (9.4%) had reactive test results and 277 (4.7%) presented with new infections. (Among the new cases, 151 (54.5%) were found in females and 55 (19.9%) were reinfections.)4
In response to the volume of syphilis cases, the WRSU implemented a public health and clinical care program that included a rapid syphilis testing component. Access to screening was expanded across all departments (e.g., main pharmacy, outpatient clinics, inpatient wards, the tribe's Division of Health and Emergency Operations Center, and the emergency department) where community health representatives underwent a three-part educational series and training on POC testing.
After the program’s implementation, infections declined 65% throughout the observation period from a peak number of 20.3 average infections per month (January–March 2022) to 7.0 infections per month by June 2023. Overall, there was a significant decline in test positivity from 5.0% in the first three months to 1.0% during the final three months of the observation period. The study illustrates how implementing a rapid POC syphilis testing program serves as an agile tool in addressing the epidemic for underserved communities.
FIGURE 2. A serologic response to infection with Treponema pallidum, the causative agent of syphilis
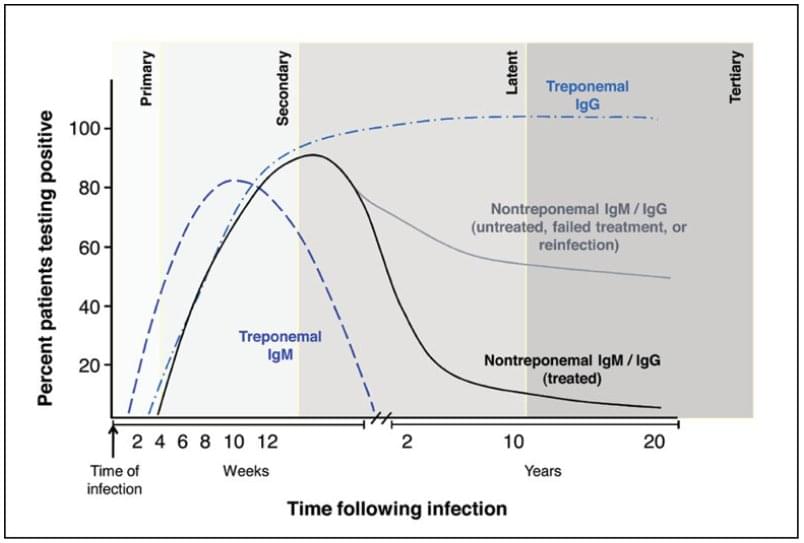
Source: Adapted from Peeling RW, Mabey D, Kamb ML, Chen X-S, Radolf JD, Benzaken AS. Syphilis. Nat Rev Dis Primers 2017;3:17073. Used with permission.
Antibody Detection
Syphilis testing is not without its challenges. Syphilis is a multistage disease classified by primary, secondary, latent, and ‘late’ or tertiary stages. Treponemal antibodies generally remain present throughout a person’s lifetime regardless of treatment. (Nontreponemal antibodies often decline after treatment.) A clinical examination and sexual history, along with both types of serological tests used in combination, are required to get an accurate diagnosis. Treponemal and nontreponemal tests -- and the associative algorithms -- have different sensitivities regarding antibody detection. (See Figure 2 for an illustrative overview.)5
Diagnostics Direct Syphilis Health Check Benefits
However, the benefits of running a rapid treponemal test first are significant, including: 1) a quicker diagnostic turnaround that allows a healthcare provider to discuss results and provide resources for care during the same visit. The 10-minute time-to-result feature of the rapid Diagnostics Direct Syphilis Health Check test, for example, helps minimize the risk of patients lost-to-follow up; 2) treponemal tests can detect primary infection at a slightly earlier stage than nontreponemal tests6. The earlier stages of syphilis are the most infectious. (It is estimated that 30-60% of the sexual contacts of individuals with early syphilis will acquire syphilis themselves7.) Earlier detection can help stop the spread of disease through expedited partner notification and initiation of antibiotic treatment (typically, benzathine penicillin); and 3) false positive treponemal tests occur less frequently than false positive non-treponemal tests8. It is necessary to verify the diagnosis with a non-treponemal test in fewer cases. This reduces costs associated with diagnostic syphilis procedures.
Capturing Coinfection
One more note regarding savings, public health professionals can easily incorporate Diagnostics Direct Syphilis Health Check testing into a streamlined workflow alongside OraSure’s suite of products, like the OraQuick ADVANCED Rapid HIV-1/2 Antibody Test and the OraQuick™ HCV Rapid Antibody Test. The population bearing the significant burden of the syphilis epidemic tend to share similar risk profiles for HIV and HCV. All OraSure POC kits offer easy, flexible, and accurate testing options for providers across diverse settings to capture coinfections.OraSure is proud to offer diagnostic devices that support the research and wellbeing of sexual health. For more information regarding OraSure's entire infectious disease portfolio, please view our Rapid Infectious Disease Products . If you are interested in case studies, webinars and other industry perspective, please visit IDX Insights.
References:
1,3,5 Papp JR, Park IU, Fakile Y, Pereira L, Pillay A, Bolan GA. CDC Laboratory Recommendations for Syphilis Testing, United States, 2024. MMWR Recomm Rep 2024;73(No. RR-1):1–32. DOI: http://dx.doi.org/10.15585/mmwr.rr7301a1.
2 Fang J, Partridge E, Bautista GM, Sankaran D. Congenital Syphilis Epidemiology, Prevention, and Management in the United States: A 2022 Update. Cureus. 2022 Dec 27;14(12):e33009. doi: 10.7759/cureus.33009. PMID: 36712768; PMCID: PMC9879571. https://pmc.ncbi.nlm.nih.gov/articles/PMC9879571/.
4 Close, Ryan M. MD, MPH∗; Weigle, Alex BSN, RN∗,†; Thompson, Trevor PharmD∗,†; McAuley, James MD, MPH∗. Integrated Response to Address a Resurgent Syphilis Epidemic in a Rural American Indian Community, Whiteriver, Arizona, January 2022 to June 2023. Sexually Transmitted Diseases 51(3):p 156-161, March 2024. | DOI: 10.1097/OLQ.0000000000001909.
6 Soreng, K., Levy, R. & Fakile, Y. Serologic Testing for Syphilis: Benefits and Challenges of a Reverse Algorithm. Clin. Microbiol. Newsl. 36, 195–202 (2014).
7 Watson-Jones D, Gumodoka B, Weiss H, Changalucha J, Todd J, Mugeye K, et al. Syphilis in pregnancy in Tanzania. II. The effectiveness of antenatal syphilis screening and single dose benzathine penicillin treatment for the prevention of adverse pregnancy outcomes. J Infect Dis 2002;186:948-57.
8 Geusau A, Kittler H, Hein U, Dangl-Erlach E, Stingl G, Tschachler E. Biological false-positive tests comprise a high proportion of Venereal Disease Research Laboratory reactions in an analysis of 300,000 sera. International Journal of STD & AIDS. 2005;16(11):722-726. doi:10.1258/095646205774763207.
OraQuick is a registered trademark of OraSure Technologies, Inc. Syphilis Health Check is a trademark of Diagnostics Direct, LLC, used under license by OraSure Technologies, Inc.