

OraSure QuickFlu® is an FDA cleared, CLIA-waived in vitro rapid qualitative test for the detection and differentiation of influenza type A and type B. An easy nasal swab procedure provides accurate results in as little as 10 minutes.
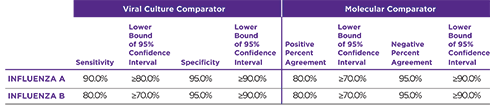
Orasure QuickFlu® delivers highly accurate results for flu types A and B and meets FDA's new standards of performance.
OraSure QuickFlu® exceeds the FDA's reclassification requirement minimums for RIDTs.
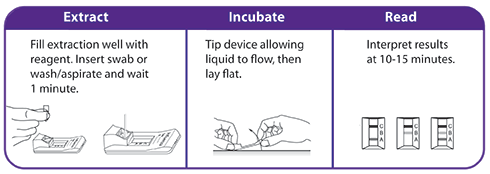
OraSure QuickFlu® is ideal for testing at the point of care in...
CPT Codes
87804 QW- Influenza A Detection
87804-59- Influenza B Detection
*OraSure provides information regarding reimbursement (including, in some instances, potentially applicable CPT, HCPCS and/or analogous state or local codes or designations) for background purposes only. It does not constitute legal advice or a recommendation regarding clinical practice. Information provided is gathered from third-party sources and is subject to change without notice. The provider has the responsibility to determine medical necessity and to submit appropriate codes and charges for care provided. Any decision regarding specific coding is at the discretion of the healthcare professional, and provision of this information does not guarantee or facilitate reimbursement. OraSure makes no guarantee that the use of this information will prevent differences of opinion or disputes with Medicare or other payers as to the correct form of billing or the amount that will be paid to providers of service. Please contact your Medicare contractor, insurance provider, other payers, reimbursement specialists and/or legal counsel for interpretation of coding, coverage and payment policies or specific billing questions or concerns. Nothing herein is intended to promote or facilitate the purchase or use of products outside of their approved or cleared indications, and appropriate use of products should be based on the healthcare professional’s medical judgment.